Pure
iron, prepared by the electrolysis of ferrous sulfate
solution, has limited use. Commercial iron
invariably contains small amounts of carbon and other
impurities that alter its physical properties, which are
considerably improved by the further addition of carbon
and other alloying elements. By far the greatest amount
of iron is used in processed forms, such as wrought iron,
cast iron, and steel. The image below shows how
iron is "cast" into forms that, when cool, will
be referred to as "cast iron". The iron
cast is made of sand, possibly
mined in Michigan.
The differences between the various types of iron and
steel are sometimes confusing because of the nomenclature
used. Steel in general is an alloy of iron and carbon,
often with an admixture of other elements. Some alloys
that are commercially called irons contain more carbon
than commercial steels.
Modern steelmaking employs blast
furnaces, like the one seen below, that are
merely refinements of the furnaces used by the old
ironworkers. The process of refining molten iron with
blasts of air was accomplished by the British inventor
Sir Henry Bessemer who developed the Bessemer furnace, or
converter, in 1855. Since the 1960s, several so-called minimills have been producing
steel from scrap metal in electric furnaces. Although
such mills are an important component of total US steel
production, the giant steel mills that create steel
directly from iron ore remain essential for the initial
production of steel.

Source: Unknown
Steel is a remanufactured product that uses pig
iron as its main raw material.
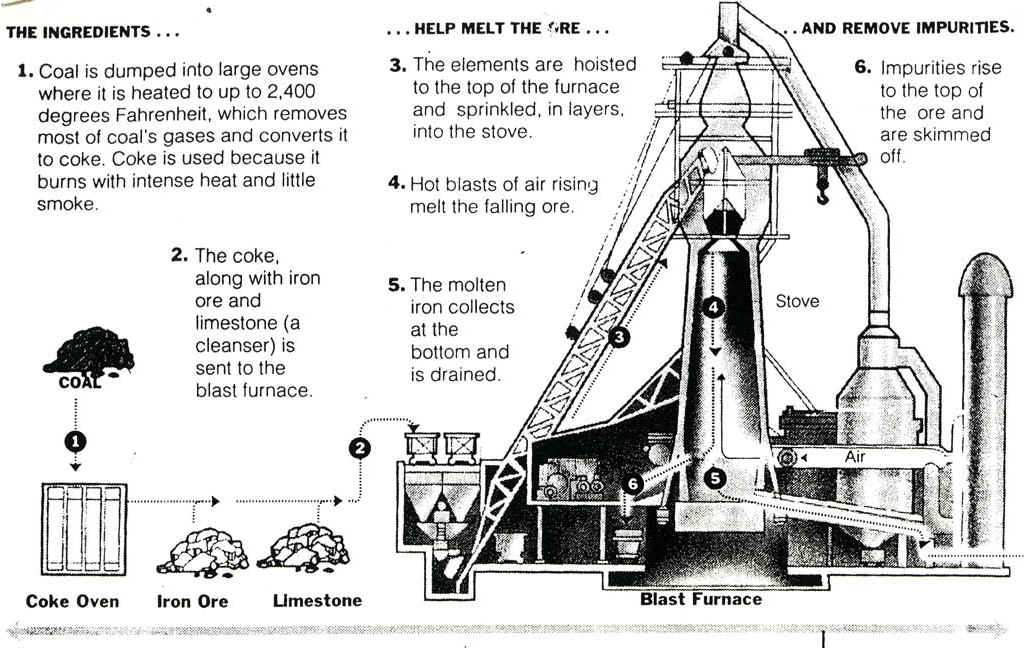
Pig-Iron Production
The basic materials used for the manufacture of pig iron
are iron ore, coke, and
limestone. Coke is the hard,
porous residue left after the destructive distillation of
coal. Used as a reducing agent in the smelting of pig
iron and as a fuel, coke is blackish-gray and has a
metallic luster. It is composed largely of carbon,
usually about 92%. When used as a fuel, it has a high
heating value of 13,800 Btu/lb.
Coke
was first produced as a by-product in the manufacture of
illuminating gas. The growth of the steel industry,
however, produced a rising demand for metallurgical coke,
making it inevitable that coke should be manufactured as
a chief product rather than as a by-product. The
earliest method of coking coal was simply to pile it in
large heaps out-of-doors, leaving a number of horizontal
and vertical flues through the piles. These flues were
filled with wood, which was lighted and which, in turn,
ignited the coal. When most of the volatile elements in
the coal were driven off, the flames would die down; the
fire would then be partly smothered with coal dust, and
the heap sprinkled with water.
Coke is burned as a fuel to heat the
blast furnace; as it burns, the coke gives off carbon
monoxide, which combines with the iron oxides in the ore,
reducing them to metallic iron. This is the basic
chemical reaction in the blast furnace; it has the
equation:
Fe2O3 + 3CO = 3CO2 + 2Fe.
The limestone in the furnace is used as an additional source of carbon monoxide and as a "flux" to combine with the infusible silica present in the ore to form fusible calcium silicate. Without the limestone, iron silicate would be formed, with a resulting loss of metallic iron. Calcium silicate plus other impurities form a slag that floats on top of the molten metal at the bottom of the furnace. Ordinary pig iron as produced by blast furnaces contains about 92% iron 3-4% carbon, 0.5-3% silicon, and trace amounts of manganese, phosphorus, and sulfur.
Cast Iron
Cast iron is a study in contradictions. It is a symbol of
strength, sturdy enough to support a massive industrial
building, and of weakness, brittle enough to shatter when
dropped. Tamed in a foundry furnace to a molten mass,
this mineral pulled from the earth flows obediently into
the intricate details of a sand mold.
As far back as 3500 BC, Egyptians
mined iron from meteorites, the only form in which it
exists as a pure element. But it took another 1,500 years
to figure out how to smelt it--extract it from ore where
it lives as an oxidized compound. Iron was probably cast
for the first time soon afterward. Europeans began
casting iron in the15th century, but the black metal
remained a rare and precious substance for nearly 300
years because melting iron required enormous amounts of
wood for fuel. In the 1600s, England went so far as to
ban cast iron production to protect its forests. Yet
ironically, it was an Englishman who made possible
iron’s modern ascendance. In 1709, he discovered
that coke, a baked coal that burns hotter than wood or
coal, could be used to efficiently smelt iron, then heat
it to the 2,800F that renders it castable.
With its 2-4.5% carbon content, cast
iron is more brittle and rust-prone than its low-carbon
(less than 0.03%) hammer-forged cousin, wrought iron. But
the casting process is better suited to mass production
than black-smithing, so the molded metal’s star rose
meteorically during the Industrial Revolution. From
frying pans to steam engines, from bathtubs to drain
pipes, cast iron had an effect on every aspect of
people’s lives. Here was a versatile, durable,
easily formable material.
Cast iron’s architectural heyday
ended with the development of steel, a stronger and more
durable material that is itself an iron-based alloy with
a very low carbon content (0.015 to 0.5%). Today’s
foundries make their cast iron mostly from recycled scrap
steel, or scrap mixed with pig iron--smelted,
carbon-infused iron. By throwing in scrap, they create a
mix or iron, carbon, and minerals like silicon and
manganese that are in modern-day cast-iron alloys.
Melted in an electric furnace, the
iron is poured into a sand mold made from wooden
patterns. Pattern making is a highly skilled trade.
Foundries employ teams of designers to create, on
computers, exact replicas of original pieces, then
hand-cut the designs in wood. The pattern is carved
slightly larger than the intended product to account for
cooling shrinkage.
The wooden pattern is set into a wood
form that is filled with clay-infused sand to make half
of one mold; to make the other half, another sand-filled
form is packed around the protruding pattern, which is
then removed. The liquid iron comes to the mold via a
crucible, a large bucket that travels on a track or crane
from the furnace. A foundry worker ladles the white-hot
liquid into the mold or, for a large cast, pours it
straight from the crucible. After the iron cools and
hardens, usually within hours, the sand cast is broken
off and foundry workers blast away the last sand grains
with metal shot. Because of its brittle nature, cast iron
shouldn’t be shaped after cooling.
The recipe for steel
Here's the recipe for a typical
"batch" of molten pig iron. For each ton of molten pig iron, you need:
2600 lbs iron ore or iron ore pellets,
1000 lbs coke,
and a few hundred lbs of flux (slag,
calcite, dolomite, limestone, etc). Calcite or
dolomite is used to make steel. In some instances, burnt
lime(manufactured by heating calcite or dolomite) is
substituted. The lime in the stone or burnt lime (when
melted in blast furnaces, basic oxygen furnaces, or
electric furnaces) combines with the impurities in the
ore or hot metal to form slag, which, because it is
lighter, floats on top of the molten metal. Take a
few minutes and "walk through" the process of
steel-making as nicely illustrated in the 12-step
diagrams below.
Note the steel mill below, with its blast furnaces, and
the raw materials for them: piles of iron ore pellets
being offloaded from the ore freighter, AND piles of
crushed limestone to be used for flux.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan State University
The image below shows a Great Lakes' freighter unloading
iron ore pellets at the Algoma Steel plant, in Sault Ste.
Marie, Ontario. Note the large piles of pellets
onshore, and the two large blast furnaces in the
background.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan
State University
A typical blast furnace consists of a cylindrical steel
shell lined with a a nonmetallic substance such as
firebrick. The shell is tapered at the top and at the
bottom and is widest at a point about one-quarter of the
distance from the bottom. The lower portion of the
furnace, called the bosh, is equipped with several
tubular openings or tuyeres through which the air blast
is forced. Near the bottom of the bosh is a hole through
which the molten pig iron flows when the furnace is
tapped, and above this hole is another one for draining
the slag. The top of the furnace, which is about 27 m
(about 90 ft) in height, contains vents for the escaping
gases, and a pair of round hoppers closed with
bell-shaped valves through which the charge is introduced
into the furnace. The materials are brought up to the
hoppers in small dump cars that are hauled up an inclined
external skip hoist.
Blast furnaces operate continuously
and are never shut down. The raw material to be fed into
the furnace (see the recipe above) is divided into a
number of small charges that are introduced into the
furnace at 10- to 15-min intervals. Slag is drawn off
from the top of the melt about once every 2 hr, and the
molten iron itself is drawn off or tapped about five
times a day. The air used to supply the blast in a blast
furnace is preheated to temperatures between
approximately 540� and 870� C (approximately 1000� and
1600� F). An important development in blast
furnace technology, the pressurizing of furnaces, was
introduced after World War II. By "throttling"
the flow of gas from the furnace vents, the pressure
within the furnace may be built up to 1.7 atm or more.
The pressurizing technique makes possible better
combustion of the coke and higher output of pig iron. The
output of many blast furnaces can be increased 25% by
pressurizing. Experimental installations have also
shown that the output of blast furnaces can be increased
by enriching the air blast with oxygen.

Source:
Unknown
The process of tapping the blast furnace (pouring out the
molten pig iron, and casting them into small rectangular
blocks known as "pigs") consists of knocking
out a clay plug from the iron hole near the bottom and
allowing the molten metal to flow into a clay-lined
runner and then into a large, brick-lined metal
container, which may be either a ladle or a rail car
capable of holding as much as 100 tons of metal. Any slag
that may flow from the furnace with the metal is skimmed
off before it reaches the container. The container of
molten pig iron is then transported to the steelmaking
shop. The machine below is a pig caster, which
accepts molten iron into rectangular receptacles, and
allows it to harden into "pigs". The pig
casting machine below makes pigs that are 10 lbs,
approximately 5" x 6" x 3" in size.
The 165 ft. (50 m.) long machine can cast 150 tons of
molten iron in about ninety minutes, for a daily capacity
in excess of 1,500 tons.
Here's another pig casting operation (below), in which
the iron is tapped into a torpedo ladle car, which then
transports it to a pig casting machine. You can see the
ladle cars, which are essentially an assembly line of
molds that move along as molten iron, here being poured
from the blast furnace, is discharged into them.

Source:
Unknown
The oldest process for making steel in
large quantities, the Bessemer process, made use of a
tall, pear-shaped furnace, called a Bessemer converter,
that could be tilted sideways for charging and pouring.
Great quantities of air were blown through the molten
metal; its oxygen united chemically with the impurities
and carried them off. In the basic oxygen process, steel
is also refined in a pear-shaped furnace that tilts
sideways for charging and pouring. Air, however, has been
replaced by a high-pressure stream of nearly pure
oxygen. The molten iron is next cast into a variety
of shapes. In our example we will be discussing how
steel that is cast into rectangular ingots or slabs (see
below) is reworked into sheet steel.

Source: Unknown
Steel
Steel is marketed in a wide variety of sizes and shapes,
such as rods, pipes, railroad rails, tees, channels, and
I-beams. These shapes are produced at steel mills by
rolling and otherwise forming heated ingots to the
required shape. The working of steel also improves the
quality of the steel by refining its crystalline
structure and making the metal tougher. The basic process
of working steel is known as hot rolling.
Hot rolling is especially applicable
to ingots like the one shown above. In hot rolling the
cast ingot is first heated to bright-red heat (2300 F) in
a furnace called a soaking pit and is then passed between
a series of pairs of metal rollers that squeeze it to the
desired size and shape. The distance between the
rollers diminishes for each successive pair as the steel
is elongated and reduced in thickness. Continuous mills
roll steel strips and sheets in widths of up to 2.4 m (8
ft). Such mills process thin sheet steel rapidly, before
it cools and becomes unworkable. A slab of hot steel over
11 cm (about 4.5 in) thick is fed through a series of
rollers which reduce it progressively in thickness to
0.127 cm (0.05 in) and increase its length from 4 m (13
ft) to 370 m (1210 ft). The rollers can generate up to 3500 tons of pressure per square inch;
5000-10,000 hp motors are used to turn the rollers.
The steel ingot is eventually passed to many
roller mills that reduce it to the correct thickness. The
rollers of mills used to produce railroad rails and such
structural shapes as I-beams, H-beams, and angles are
grooved to give the required shape.
Modern manufacturing requires a large
amount of thin sheet steel. Think of all the
products you use every day that have sheet steel in them:
cars, appliances, buildings, desks, trucks, tables, cans,
boxes, etc! they all started out as rolls of steel
like the one shown below.
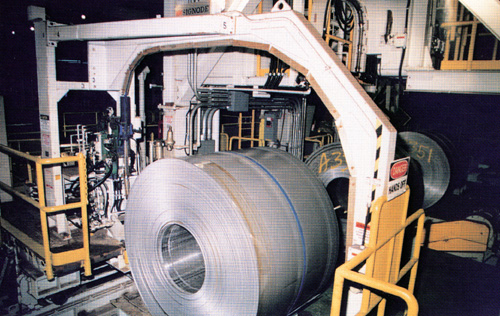
Source: Unknown
Many of Michigan's steel mills produce primarily rolled steel. The steel is rolled "cold"--that is, it is rolled at room temperature and not at high temperatures. Eventually, the optimal thickness of the steel is achieved, as at the exit reel area.
And finally, the steel is "on the road", on
its way to an automobile body or assembly plant.
Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan State University
Parts of the text on this page have been taken from "This Old House" magazine.
This material has been compiled for educational use only, and may not be reproduced without permission. One copy may be printed for personal use. Please contact Randall Schaetzl (soils@msu.edu) for more information or permissions.