IRON MINING: WHERE AND WHY?
It was called the barren waste, this land that is now Michigan’s
Upper Peninsula. However, since its first iron ore shipment of two hundred pounds in 1846,
the Upper Peninsula mines (below) have produced well over one billion tons of iron ore.
Some barren waste!
Iron ore is Michigan’s most valuable non-fuel, mined commodity. In
1994, the Lake Superior area produced 95% of the US’s supply of iron ore. Minnesota
leads the nation with 70% of the production, while Michigan produces most of the rest
(25%). More than 98% of the iron ore that is shipped in the world is used to make steel,
while the rest is used in pigments, chemicals, or in other, minor uses. Natural ores were
the primary type of ores mined prior to World War II.
In the early 19th century, settlers moving westward from the eastern
United States bypassed the UP because of its reputation as an inhospitable wilderness.
However, favorable attention was drawn to the region by Douglass Houghton’s 1841
geological report describing the presence of copper on the Keweenaw Peninsula. Adding to
this excitement was the publicity surrounding the delivery of a two ton Ontonagon copper
boulder to Detroit in 1843.
During the first part of the Precambrian era, a 250 million year-period
of quiet, which we name the Huronian from its record north of Lake Huron, thick sediments
were laid down in a shallow sea trough that covered the Lake Superior region. In places
thick sand was deposited; in other fine muds, and in other places pure lime, accumulated
in the shallow but slowly deepening sea. Over the sand great masses of iron minerals
accumulated, either by chemical action or by the work of iron forming bacteria, or by both
and perhaps other means, until vast thicknesses of sand and iron sediments were built up,
and the world’s largest iron deposit was in the making in Minnesota, Wisconsin, and
Michigan. In that far ago time the foundations of Michigan’s wealth and the
automobile industry were laid in the old Huronian sediments we now find in the iron ranges
of Marquette, Baraga, Iron, Dickinson, Menominee, and Gogebic counties.
Huronian (Early Proterozoic) rocks hold the vast wealth of the
iron formations in Michigan. As we have found, those rocks are broken, faulted, some even
turned over backward. They are so cracked and shoved about, that the edges of ore bodies
are separated by hundreds of feet, which makes mining difficult and the search for ore
bodies complicated. But since its discovery in 1844 Michigan has mined one of the richest
iron ore fields in the world, and for more than 100 years the wealth and industry of the
state has depended in large measure on iron ore.
The term "iron-formation" is perhaps the most enigmatic and
controversial term ever concocted for a rock type. Iron formations were deposited in many
parts of the world during the Early Proterozoic (Precambrian) in formations that are 500
to 2,000 feet or more thick and that extend for hundreds of miles. Therefore, thousands of
cubic miles of iron-rich sediments were deposited in a number of basins. After the Early
Proterozoic (about two BILLION years ago) deposition of iron-formation ceased, and this
distinctive rock type is essentially absent from younger geological sequences.
Thus, we are left to puzzle over where the vast quantities of iron came
from during the Early Proterozoic, and why iron is not found in younger rocks. This all
suggests that a major change in the chemistry of the surface environment of the
earth must have occurred around two billion years ago to prevent iron-formation deposition
in younger rocks. Most geologists now believe that the major change was the development of
an oxygen-bearing atmosphere on earth. Iron is relatively soluble in an
oxygen-deficient environment (like that of the earliest Precambrian) and therefore would
be present in large amounts in ocean waters. In oxygen-rich environments, like the present
atmosphere, iron is incredibly insoluble, and therefore is not present in quantity in
ocean waters.
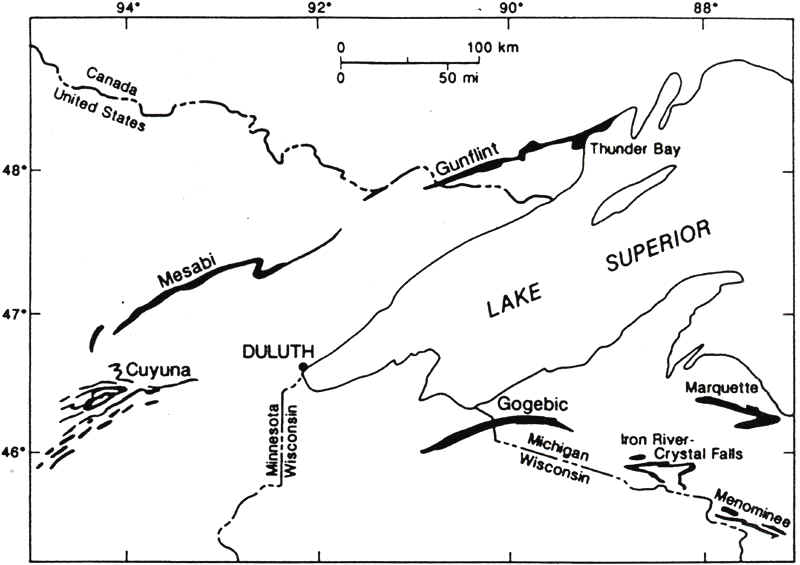
Iron-formations have played an important part in the settlement and
economic development of the Lake Superior region, for these rocks are the source and host
for a vast majority of the iron ore produced in North America. The map below shows
that iron formations are commonplace throughout the Lake Superior region. The
largest of these is the Mesabi Range in Minnesota.
Source: Unknown
And today, land devoted to mining in the Great Lakes region is dominated by the large,
open-pit mines in the Mesabi Range of Minnesota.
Source: Unknown
Iron ore was first "discovered" by European settlers when the
early land surveyor, William Austin Burt, was shown iron ore by an Indian chieftain near
what is now Negaunee, Michigan, in 1844. He had noticed a strange movement of his compass
needle while surveying near where the present town of Ishpeming is located.
Mining commenced in 1846, grew steadily, and has continued to the
present time. Discovery of iron ore in other parts of the Lake Superior region constitutes
one of the most colorful chapters in the history of the Great Lakes area. Mining was
second only to logging in bringing in new people and in opening the area to settlement.
Its importance, however, has far outlasted that of logging, for it is still a major
contributor to the economy of the region.
The iron ores were found in near surface zones of enrichment
in Precambrian rocks of the northern Great Lakes states. At the peak of WW II, 103 natural
ore mines were functioning in Minnesota and 43 were running in Michigan. These ores
averaged about 60% iron. They could be shipped directly to the steel mills without
"beneficiation" (the process of crushing, screening, drying, washing, and other
processes that separate the ore particles from the worthless silica that is intimately
intermingled with it in low-grade ores).
Iron-formation was first studied in the Lake Superior region in the
late 1800's. In fact, iron-formations in this region are the "standard" to
which others throughout the world are compared. Although Michigan has many iron-rich
rocks, usually the amount of iron in them is so low as to preclude mining. In iron
formations, the amount of iron has been concentrated by weathering and leaching of
non-iron minerals, such that we only use the term "iron formation" for rocks
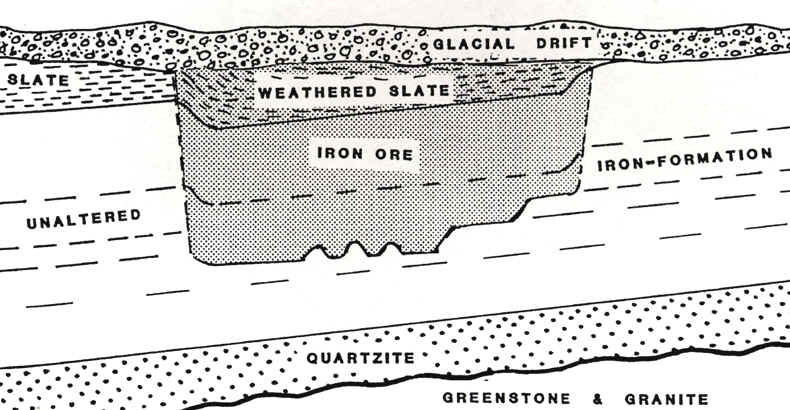
that contain >50% iron. The ore (iron) is concentrated by downward percolating
water, and occurs in places where downward flowing waters are guided and converge, such as
along a slanting layer of impermeable rock below. Along these channels silica is
removed and iron oxides are concentrated. Leaching is more efficient in places where
the original sedimentary rock (containing thin layers of iron-rich materials) are bent,
folded and broken, acting like a sieve and not a barrier to infiltrating water. The
diagram below illustrates the relationship between unaltered "iron formations"
and the weathered, leached iron ore, as they are found in the many iron ranges of the UP
and Minnesota.
Source: Unknown
This time of iron formation occurred in the long interval between the Jurassic (about 200
million years ago) and today. During this time, few rocks were actually forming in
Michigan. Instead, all the forces of erosion were at work creating much of our
scenery, exposing our mineral wealth, and creating some of it. The old Precambrian area
west of Marquette was eroded most rapidly. The edges of the Cambrian sandstones were worn
away off the old granites and Huronian iron formations, leaving only small caps of
sandstone to show where the Cambrian seas had been. The iron formations were not ores when
first exposed, but during the long ages of their exposure surface waters leached out other
minerals leaving the iron behind, gradually changing the upper few hundred feet iron ore,
or what the miners called "direct shipping ore". That explains in part why the
upper parts of the iron formations are rich and the lower levels lean in iron.
Although iron-formation has a simple composition---it is composed of
chert (biochemically precipitated quartz) and iron minerals---it is extremely variable in
appearance. Both the chert and iron minerals are varied in color. Despite these
variations, iron-formations have distinctive features that provide a basis for
identification. As the name implies, they are rich in iron minerals, with one or more
iron-bearing minerals constituting nearly half of the volume of the rock. Iron
formations are thinly-banded and are of chemical-sedimentary origin.
Source: Unknown
Iron-formations form distinctive sedimentary rocks in greenstone
belts in Archean-age rocks. Iron-formations consist mainly of chert (chemically
precipitated quartz) and iron-rich minerals. In some units the rock may be mainly
chert; in other it may be chert-hematite or it may be jasper-hematite, which is a
spectacular rock with alternating red and gray layers, each about 5-20 mm thick. Chert and
magnetite are by far the most common minerals in the iron-formations. Other iron minerals
include siderite and hematite. The image below shows a few specimens of iron
ore--iron formation. The gray metallic ore is magnetite, while the red ore is
hematite. The round thing in the middle is a quarter.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan State University
Some iron-formations seem to have formed by volcanic hot springs
contributing iron and silica to sea water, probably in basins between volcanic islands.
The silica and iron was probably precipitated by biochemical processes.
Some iron-formations have highly carbonaceous layers (now mainly
graphite) associated with them. Indeed, carbonaceous shales and graphitic layers are
common in both the sedimentary and volcanic rocks of greenstone belts. Studies of
these carbonaceous layers indicate that they are composed mainly of organic carbon.
This would attest to the presence of primitive organisms (probably blue-green algae and
bacteria) in amounts great enough to accumulate in layers many feet thick in the waters in
which the greenstone belts formed. It also points to the antiquity of life on earth, for
the oldest greenstone belts yet studied (at least 3,300 million years old in South Africa)
have evidence that primitive life was abundant enough to leave a record.
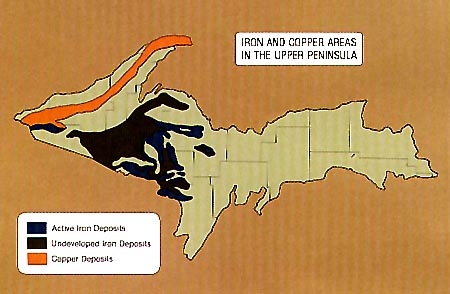
Iron was located in three of four areas in the Precambrian rocks of the
western UP. The map below outlines the locations of Michigan's major iron ranges.
These iron ranges are the roots of middle Precambrian mountains.
Of the six principal iron ranges, or areas, in the United States, three are
located primarily in Michigan: the Marquette Range, all of which is found within the
state, and the Menominee and Gogebic ranges which are located in both Michigan and
Wisconsin. Approximately 40 miles long and 3-10 miles wide, the Marquette Range
stretches across the UP from the city of Marquette to a few miles south of L'Anse on the
Keweenaw Bay. The Gogebic Range lies partly in Michigan and partly in Wisconsin. It is
divided by the Montreal River, a short stream that flows into Lake Superior about 25 miles
east of Ashland, WI. This range extends almost 80 miles between Atkins Lake in Wisconsin
and Lake Gogebic in Michigan; the Michigan section is approximately 25 miles long and
stretches from the state boundary at Ironwood to a point slightly west of Lake Gogebic.
The greater portion of the Menominee Range lies in the state of Michigan and includes the
towns of Iron Mountain, Norway, and Vulcan. Main iron deposits in this range extend in an
east and west direction, north of Iron Mountain.
If iron and steel manufacturing happened to be localized by its ore
sources rather than by coal sources or markets, suffice it to say that the economic
development and history of the Upper Peninsula would have been much different than it is.
At first the iron ore was smelted in charcoal furnaces near
Marquette---one of the old furnaces is now a tourist exhibit. The charcoal was made from
the "inexhaustible" hardwoods of the original forest, but as the hardwoods were
depleted and newer methods of smelting came into use it became cheaper and more expedient
to move the iron ore to the fuel---the coal of Pennsylvania. A few furnace towns were
abandoned and their buildings left to crumble and stare blankly at the brush growing in
the streets.
The Marquette Range
First to be discovered, the Marquette Range had been of interest to geologists
since the early 1840s when Douglass Houghton, Michigan's first State Geologist, conducted
a systematic scientific analysis and exploration of Michigan's Upper Peninsula. He
published his findings in a lengthy report, detailing the locations of minerals in the
Lake Superior area. Although he was not aware of the quantity of iron ore deposits in the
area, Houghton did state that iron deposits were to be found on or near the south shore of
the lake. Houghton's findings were substantiated and enlarged by the activities of William
A. Burt, a United States Deputy Surveyor. Burt, in attempting to establish the east-west
line between townships 47 North and 48 North, approximately one mile south of Teal Lake,
noted strange variations in his magnetic compass needle. When using Burt's own invention,
the solar compass, no variations were observed. Searching for the cause of this
disturbance, the surveying party discovered deposits of iron ore, or hematite. In 1845,
the search for iron ore began in earnest, and the first major discovery was made near the
present site of Negaunee. The men of the search party formed the Jackson Mining Company on
July 23, 1845, and iron mining in Michigan officially began. A producing wrought iron from
ore was erected on Carp River, and it was there that the first metallic iron was made.
The first blast furnace, built near the Jackson Mine, went into operation in April
of 1849.
The Civil War increased the need for Lake Superior iron, and the volume
of shipment began a steady increase, with additional mines opening on the range. In 1861,
the output for the Marquette Range was 120,000 tons; by 1868, annual figures had reached a
half million tons; and, by 1873, the range produced over one million tons of ore, a figure
that steadily increased through the turn of the century.
Source: Unknown
The Menominee Range
Before the Civil War, the major iron-producing region was the Marquette Range, but the
postwar discovery of iron in the Menominee and Gogebic ranges greatly expanded
Michigan’s production capacity.
In 1846, William A. Burt, in the process of his linear land survey,
noted signs of iron ore in the Crystal Falls area. In May of 1849, J. W. Foster was sent
by Dr. Charles T. Jackson to investigate the reports of iron ore on the Menominee River,
and Foster's subsequent report listed large beds of specular ore in Section 30, T40N,
R30W, near Lake Antoine. In 1866, Thomas and Bartley Breen, timber speculators from
Menominee, located iron deposits near present Waucedah, Michigan. Although the Breen
brothers had discovered what later became the very profitable Breen Mine, actual mining
operations on this location did not commence until 1870. In 1878 five mines were
actively engaged in shipping from the Menominee Range: the Breen, Cyclops, Norway,
Quinnesec, and the Vulcan.
With the 1855 completion of the Soo Locks, the early lakeside mines
were able to easily transport the bulky ore to smelting sites and markets, but as
operations moved inland, the need for reliable, efficient, internal improvements became
apparent. Railroads seemed to provide the solution. Construction began in the late 1850s,
and the first interpeninsular line was completed in 1881.
Production reached its peak in 1920 when it shipped almost seven
million tons of iron ore. Decline began in the 1930s, and today crumbling ruins are almost
all that remain of most of the Menominee mines, once bustling centers of activity and
production.
Source: Unknown
The Gogebic Range
The first official recorded notice of the presence of iron ore on the Gogebic Range was
included in the 1848 report of Dr. A. Randall, who saw exposures of iron ore approximately
halfway between Hurley and Mellen, in Wisconsin. Attention was drawn in Michigan to the
possibilities of the Gogebic Range by the report of the Geological Survey for the State of
Michigan. Professor Raphael Pumpelly and Major T. B. Brooks traced the iron formation
across the Montreal River into Michigan and, in later years, mapped the range extending
eastward toward Lake Gogebic. Official credit for the discovery of iron is given to
Richard Langford, a trapper and hunter, who claimed to have seen red ore outcropping from
the roots of an overturned tree south of the present city of Bessemer in 1879 or 1880.
By 1884, this location, known as the Colby Mine, began to produce commercial iron
ore. The early explorers and prospectors on the Gogebic Range experienced the
particular hardships of a land devoid of roads or even passable trails. Supplies had to be
packed overland from Ontonagon or were brought by boat from Ontonagon to Ashland to the
mouths of the Montreal and Black rivers. However, soon after the opening of the range
for mining, the railroad made its initial entrance. The first ore from the range
came out of the Colby, and 1,022 tons were loaded onto flat cars in October of 1884, sent
to Milwaukee, and then transshipped by barge to Erie, Pennsylvania. By 1910, three
separate railway lines served the Gogebic Range, and approximately four million tons of
iron ore were being shipped annually. From 1893 through the first decade of the 20th
century, production levels ranged from 3-4 million tons of ore, and the Gogebic continued
to be a productive iron range.
The region near Ishpeming, in the heart of the Marquette Iron Range, was chosen during the mid-20th century because trees do not block the view of the iron-rich hills. Today, regrowth of forest has occurred. The hills are composed of iron-rich rocks like diorite, and other "iron formations".
We celebrate the 1844 discovery of Michigan’s iron ore
deposits, but we should really think of it as the "final" or
"commercial" or "official" discovery of this resource. For thousands
of years before the European discovery of North America, the continent’s prehistoric
inhabitants knew of and made use of native metals and metallic ores. It is ironic that the
American discoverers of what became the Jackson Mine (near present-day Negaunee) were led
to the site by a local Native American. The technologies Native Americans employed and the
resulting products were vastly different from those the Europeans would introduce.
Industrial-scale usage of metals never took place during the prehistoric period. However,
metals held special significance in the spiritual and ritual lives of Native Americans
that did not exist in the Euro-American world.
Using a combination of the traditional techniques of cold-hammering,
annealing (reheating), riveting or abrasion, Native Americans were able to craft metal
artifacts of remarkable ingenuity-and often of great beauty-from gold, silver, copper and
galena. Their use of iron is probably not so well known. Iron is one of the most common
elements in our environment and ourselves. It is the "hemo" in the hemoglobin of
our blood. However, it rarely occurs in a pure, or native, state. Nevertheless,
prehistoric people made use of iron in its many impure forms.
Taconite, a common form of iron chemically bound with silica, was
chipped and flaked into projectile points. Marcasite, or iron pyrite, was ground into
cones and hemispheres. Hematite, the richest ore source for most of the iron mined on the
American continent, was pecked and ground into celts, axes, cones and plummets. Just as
often, Native Americans were searching for the brilliant red oxide, a ready source of
pigment. An unlikely source of iron used prehistorically was meteorites. Decorative items
such as earspools, beads and buttons, as well as small chisels shaped like the incisors of
beavers found in Ohio burial mounds, were pounded out of meteoric iron brought from
Kansas.
True metallurgy and the reduction of ores to pure metals or alloys
required a level of technology not reached in pre-Columbian North America. Most
prehistoric cultures were capable of generating temperatures high enough to melt lead and
occasionally copper but never iron.
Click here to continue on with part two this page. There's a lot more!
Some of the images and text on this page were taken from various issues of Michigan History magazine.
This material has been compiled for educational use only, and
may not be reproduced without permission. One copy may be printed for personal
use. Please contact Randall Schaetzl (soils@msu.edu)
for more information or permissions.