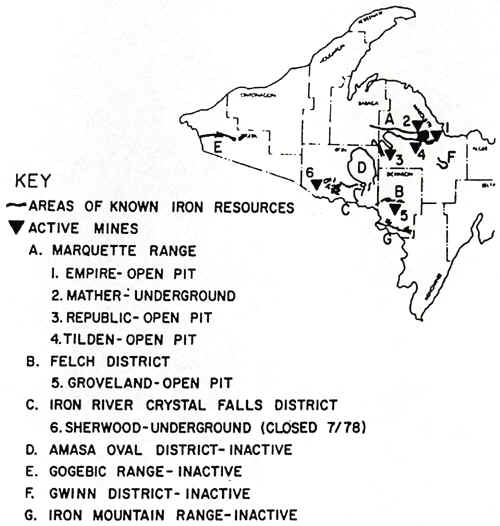
IRON MINING
Iron Ore vs "Iron Formations"
One of the most important events in the economic development of the
Lake Superior region was the (natural) conversion of parts of the iron-formations into
naturally formed iron ore bodies. The alteration of the original rock to iron ore involved
two processes that probably occurred at essentially the same time: (1) oxidation of the
iron minerals caused by waters circulating through the rocks (The soluble ferrous iron in
the minerals was converted to insoluble hematite, geothite, or limonite). and (2)
Continued circulation of water through the rocks gradually dissolved the silica from the
iron silicates and the chert and carried it out of the rock. This leaching of silica
amounted to removal of nearly 50% of the original volume of the rock. As a result, the ore
was very porous and unable to support the weight of overlying rock layers, which slumped
into ore bodies. The removal of silica thus allowed an increase in the iron content from
an initial 25 to 35% to about 55 to 60% in the ore bodies. Mining of these ores was
initially carried out in open pit mines, as shown below. The image below is of the
Jackson Mine, near Negaunee.

Source: Unknown
Later, when the easily-mined ores (those near the surface, see below) were exhausted,
deep shaft mines were required.
The large areal extent of the ore bodies and their close relationship
to the present erosion surface indicates that they probably formed by the circulation of
groundwater through the rocks. The largest of the natural ore bodies was mined as a large
open pit near Hibbing, Minnesota. It has an irregular outline but is nearly 3 miles long,
2 miles wide, and up to 500 feet deep. Nearly 200 million tons of iron ore were removed
from this mine over a period of about 50 years.
Ore bodies in the more deformed iron-formations of the iron ranges on
the south shore of Lake Superior have no obvious connection to the surface, and must have
been formed by a different mechanism. On the Menominee range, ore bodies formed in troughs
created by folded sedimentary layers. Impervious shales form the bottom of the ore bodies.
Many of these ore bodies do not extend to the surface and thus the iron-formations are
sufficiently deformed so that it was necessary to utilize underground mining methods
rather than the large open-pit operations as on the Mesabi (Minnesota) Range. Since
underground mines cannot be mechanized as can surface mines, they have been unable to
compete economically, and all have closed.
The first Michigan iron ores to be mined were soft ones (hematites), and
they were mined near the surface.
Source: Unknown
These ores, however, were soon exhausted, and mines were developed that were 3,000 to
4,000 ft (914 to 1,219 m) deep.
Mining was a slow, laborious task. It was undertaken in dark,
cold, wet mine shafts. Like copper mining, iron mining brought many ethnic peoples
to the Upper Peninsula, among them Irish, Welsh, Cornish, Italians, Swedes, Danes,
Norwegians, Finns, and French Canadians. So many immigrants flocked to the region that the
mining counties had the largest foreign population in the Upper Peninsula in the late
1800s: 12,000 in Houghton County, 10,000 in Marquette County, and 8,000 in Gogebic County.
The shaft below looks dark, cold, and wet, but recall that it has had lights installed on
the ceiling for tourists; in the 19th century such lights would have been small candles or
lanterns--and the whole shaft would have been much dimmer.
The image below shows how rocks were "hand drilled" by pounding a steel stake into the rock.

Source: Unknown
Cars like these (below) were used to haul the ore from the mine to the surface.
Source: Unknown
Source: Unknown
Click here to continue on with this page. There's a lot
more!
Some of the images and text on this page were taken from various issues of Michigan History magazine.
This material has been compiled for educational use only, and
may not be reproduced without permission. One copy may be printed for personal
use. Please contact Randall Schaetzl (soils@msu.edu)
for more information or permissions.