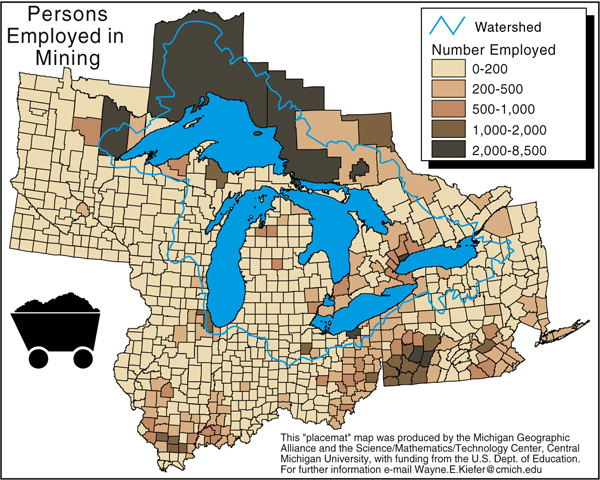
IRON MINING TODAY
Open pit mining for iron ore replaced shaft mining very early on in the history of the
Michigan Iron Ranges.
Today, all the underground mines are closed, and only a very few open
pit mines are still operating. What remains of the underground mines is often
only a few heaps of waste rocks (below) and some slumped landscapes.

Source:
Photograph by Randy Schaetzl, Professor of Geography - Michigan State University
In other areas, abandoned mining equipment stands as a reminder of past glory.

Source:
Photograph by Randy Schaetzl, Professor of Geography - Michigan State University
Where the mines were large, lakes have formed in the collapsed or slumped land. The
lake below can be crossed on Highway US-2 (in the background) in Iron Mountain. It
is a collapsed underground iron mine.

Source:
Photograph by Randy Schaetzl, Professor of Geography - Michigan State University
Many abandoned open pit mines also dot the landscapes of the UP. The image below
shows the ore silos of an abandoned open pit mine near Felch (north of Iron Mountain).
Grasses and weeds have overtaken the parking lot of a once very busy mine.
For all intents and purposes, this mine sits isolated and abandoned like a ghost town
amidst the forests of the UP.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan State University
Taconite.
Iron is one of the most vital resources of our industrial way of life,
whether in war or peace. Historically, more than 85% of the US iron ore consumed during
World War II had come from the rich open-pit hematite mines in the Lake Superior region.
By the end of the Vietnam War, however, most of the high grade ores
in the Great Lakes region had been mined, and the industry had to turn to beneficiation of
lower grade ores. Iron ore pellets now account for 97% of the US ore production.
In 1947, however, the "end" appeared near. President of
Republic Steel, Charles White, calculated the expected life for domestic iron ore using
the famed Mesabi Range in Minnesota as his benchmark. "At the present consumption
rate" White predicted, "the Mesabi will be exhausted within 5-10 years."
High grade ore, originally so plentiful on all three iron ranges, became less
available and less profitable to mine. The cost of underground mining became more
prohibitive, and the owners of Michigan's mines began to search for other methods of
tapping the iron riches of Lake Superior. Between World War I and World War II, the total
tonnage of iron ore shipped fluctuated drastically: in 1920, Michigan shipped over 18
million tons of ore. In 1921, 1931, 1932, 1933, and 1934 Michigan shipped less than half
that amount.
In response, the steel industry then launched a dual attack to expand
iron ore reserves. First, it sought new deposits of high-grade ores in foreign countries.
Second, efforts were launched to develop new technologies capable of enriching the iron
ore content of the abundant but traditionally uneconomical, low-grade taconite ores in the
US. The US government enacted policies supporting these industrial efforts, especially the
development of new taconite technology. Within a few short years, both attempts succeeded
in reducing the iron ore shortage, such that, by 1955, newspaper headlines and magazine
articles were publicizing a different tale: "Depletion Danger Met," "Worry
Over Predicted Shortage of Iron Ore Can Be Forgotten" and "One More
‘Scarcity’ Ends for US Industry." Today supplying domestic iron ore to US
steel mills is not a problem. If anything, the situation is one of oversupply!
Naturally-existing ores ("direct shipping ores") in these ranges
produced most of the iron until 1955; since that time, most of the ore has been obtained
from beneficiation (concentration) of low-grade iron-ore deposits. There were five active
iron mines in 1979 in the Marquette and Menominee ranges, but one of these five--the
Mather mine--closed in July 1979.
Rocks with about 50% or more iron constitute natural iron ore, but as this
ore became depleted, methods were developed to profitably mine lower-grade iron
formations, called taconites, which have 25-30% iron content. The taconite is crushed and
the iron separated out through gravity and flotation processes. The fine iron particles
are agglomerated through heat and molding before being shipped. This is an expensive
process, but it brings the product up to about 60-65% iron and makes it profitable to
transport. Competition with foreign producers of high-grade iron ore continues to be a
cost problem.
It wasn’t discovering foreign ores that relieved the iron ore
depletion fear. Instead, it was the development of iron ore pellets, which today account
for more than 80% of the iron ore fed to American blast furnaces. Most hematite (a form of
rich iron ore) still used, whether foreign or domestic, is also enriched and agglomerated
into various forms similar to taconite pellets. The end result was a superior
product---pelletized iron ore.
Throughout the Lake Superior region the mining of natural ores has been
phased out in favor of mining and mechanical concentration of taconite. Taconite is
iron-formation that has not been oxidized and enriched by natural processes. Two factors
have contributed to the demise of the mining of natural ores: (1) natural ore bodies have
been largely mined out, and (2) blast furnaces are more efficient when using processed,
rather than raw, ores. The tremendous consumption of iron ore during World War II, when
the Lake Superior region was the major producer of iron ore for the Allied forces, was the
zenith of production of natural ores. A rapid decline in the mining of natural ores was
accompanied by major technological advances that led to the development of the taconite
industry. By 1950 the industry knew how to concentrate the low-grade taconite ore into
higher-grade forms, but not how to pelletize it. When the Korean War broke out in 1950,
fears of domestic resource exhaustion were rekindled. Under the Defense Production Act of
1950, Congress quickly enacted loan guarantees, rapid tax write-offs and other tax
policies that helped specific industries. This act was designed to "encourage the
exploration, development and mining of critical and strategic minerals and metals."
It worked. Thus, the biggest problem the iron ore industry faces today is that attempts to
produce more iron are working too well. Today we are far closer to a glut than a scarcity.
Technology’s solution of pelletizing low-grade taconite is the most intriguing. It
produced results that few had expected—results that scientists have called
counter-intuitive. It made low-grade ore better than the high-grade ore once used. And it
did so in a way that saved energy and labor.
Powerful magnets could concentrate pulverized lean ore of 20-30% iron
into a rich concentrate of 60-64% iron, but it could not be shipped or fed into a blast
furnace in this "baby powder" form, because it would simply blow out the top
(the furnaces have tremendous updrafts due to the high temperatures within). Thus,
the last real problem was agglomeration—making the fine pieces of concentrated ore
stick together. The agglomeration problem was solved by mixing water and clay with the
taconite concentrate before balling, then kiln firing the soft "green" balls
into hard pellets.
Natural ore bodies varied considerably in composition from place to
place, and these differences had to be dealt with in the smelting (i.e., in the steel
mill) by adding various ingredients to remove the undesired constituents. In contrast,
processed taconites are mined from large pits covering many square miles and are blended
in the mining, crushing and concentrating so as to provide the smelters with a uniform
product. In effect, much of the metallurgy in iron and steel production is now done in the
mines.
The taconite process of concentrating iron from low-grade
iron-formation has been made possible by very large-scale operation and mechanization.
Because of the low value per ton of iron ore, it is necessary to handle many millions of
tons of rock as economically as possible. Specialized techniques for drilling the ore,
special blasting techniques, huge equipment to haul and crush the ore, and equally large
concentrators to extract the iron from the waste rock are some of the more important
developments. Problems then arise concerning the disposal of the huge quantities of waste
materials. The costs to undertake this type of mining are monumental. Therefore, to
initiate a taconite operation, many hundreds of millions of tons of ore must be accessible
to mining.
Basically the taconite process involves crushing the rock to a
fine-grain size, then mechanically removing the iron minerals from the waste material.
Magnetite can be removed from the siliceous waste materials by large electromagnets.
Hematite requires a more sophisticated process for concentration. It may be selectively
removed from the waste by a process called flotation. After the iron minerals are
concentrated, they are washed to remove the fine dust adhering to the grains. Because the
remaining fine powder of iron minerals cannot be handled readily, it is necessary to form
it into pea-sized "pellets" that can be transported to and handled at the blast
furnaces. The pellets are formed by adding a small amount of bentonite clay and water to
the iron concentrate to bind the iron particles together. Tumbling this mixture in large
cylinders of inclined drums produces balls that are then hardened by firing to enable them
to withstand handling and shipping. The reserves of taconite are immense, and will provide
the basis of an iron industry that should far outlast that of the natural ores.
Too much iron ore?
There was, and has always been, lots of taconite ore available.
Taconite, the rock surrounding the naturally concentrated pockets of high-grade ore, was
the original iron-bearing rock formation and extended much further than the small pockets
of naturally concentrated ore. Pelletization allows these low-grade ores to be
mechanically concentrated and reformed at the mine site into high-grade ores suitable for
blast-furnace firing. The advent of pelletization on a commercial scale in 1955 thus
initiated the profitable use of the taconite ores.
While expanding the supply, pelletization reduced the cost of iron ore
in spite of the inferior grade being used. There were several reasons for the cost
reduction. First there was substantially less energy used. The shift in ore
technology toward pelletization produced net energy savings of at least 17%, despite the
fact that the pelletization process itself required more energy. The reduction came from
the discovery that the blast furnaces could be operated more efficiently using
pelletization inputs than by using traditional ores. The pelletizing process also reduced
labor requirements per ton by some 8 %, by increasing blast-furnace output.
In general, pelletized taconite iron ore involves the substitution of
mechanical energy for chemical energy. As a result of this substitution, there is an
overall net energy saving. The mechanical energy required for pelletizing includes
grinding, magnetic separation and forming pellet balls. These pellet balls are then fed to
the blast furnace. There, coke made from coal as well as natural gas, recycled off-gasses
and occasionally other energy sources are mixed with the ore burden and its fluxing
agents. The energy sources are ignited to melt and chemically reduce the iron oxide in the
blast furnace burden to pure iron.
Chemical reduction is a complicated process, but it is more efficient
with taconite pellets, which have less silica and other undesirable elements chemically
removed and a stronger, more permeable structure that allows the hot air to move
throughout the blast furnace more efficiently. When considering the changes in all direct
and indirect energy requirements per ton of molten iron, the total energy requirements
decline dramatically with increased taconite pellet use.
Taconite pellets simply work better in blast furnaces than the soft,
red ores. Pellets are the preferred form of iron ore because they improve productivity,
conserve energy and allow greater quality control of iron production from the blast
furnace. Total costs per ton of molten iron are less with pellets than with naturally
concentrated iron ore. It’s that simple. By developing the pelletizing process, iron
miners have made most of the high-grade red ores obsolete. Technology won the battle
against a resource scarcity.
Transporting the ore to the ore docks, and then down the Great Lakes
Come all you bold sailors that follow the Lakes
On an iron-ore vessel your living to make.
I shipped in Chicago, bid adieu to the shore,
Bound away to Escanaba for red iron ore.
Most of the iron ore mined in the UP leaves the region from the ore
docks at Marquette or at Escanaba. This has been true from the very beginnings of
mining. This image below shows an early view of Marquette--even then it had a dock
that was capable of being used to load freighters with iron ore.
However, by 1861, iron ore was being regularly shipped out of the Marquette harbor (the
Soo Locks opened in 1855), and thus it's dock took on a different look. It
was now capable of loading ships with iron ore.
Today, iron ore travels by train from the mines to each of these two main docks.
Getting the iron ore to the ore carriers (Great Lakes freighters) is
the work of railroads. In the iron ore district of the Upper Peninsula, one of those
railroad lines was (and still is) the LAKE SUPERIOR & ISHPEMING RAILROAD COMPANY. Some
of the history of that line, taken from an internal document of theirs, is reported below.
All of the raw ore from the Marquette Range is mined from open pits.
Concentrators and pellet plants, which produce on a year-long basis, concentrate and
pelletize the raw ore. During the closed period of navigation, pellets are stored in
stockpiles at the plants and later loaded by large front-end loaders during the following
navigation season. During the normal shipping season, the daily production of the pellet
plant is loaded directly into ore cars for transporting to our dock at Marquette or for
interchange shipment to the Lake Michigan port of Escanaba with the Wisconsin Central
Railroad. To complete the annual iron ore shipments before freezing weather arrives is
desirable but seldom accomplished. Temperatures frequently drop well below the freezing
mark in early November; and mixed with the snowfall, the pellets will freeze when left in
the dock pockets for a period of time which makes end of season vessel loading both
difficult and time consuming. Therefore, the shipping season, dependent upon ice
conditions in the Great Lakes, is ordinarily open from March 25 to January 15 of the
following year. A United States Coast Guard ice breaker, the Mackinaw, was placed in
service in December 1944 and helps make possible the long navigation season.
A westbound train will normally consist of 120 empty ore cars hauled by
2 diesel electric locomotives coupled in multiple series. In 1897, the contents of the
first trainload of ore was 900 long tons; now with present equipment, it is approximately
8,400 long tons per train, for a net increase of 7,500 long tons per train or 833%.
A wooden ore dock was built in 1896 in Presque Isle Harbor, Marquette,
of the latest type of construction for that period. It was 54 feet high, 1200 feet long,
with 200 pockets each holding 160 tons or a total storage capacity of 32,000 tons. By
1910, storms had made it obsolete and expensive to repair; and it was replaced by a
concrete ore dock with rolled steel pocket fronts. The approach to the new dock is about 1
mile long on a 1 � % grade and consists of an earth embankment containing 600,000 cubic
years of earth and a 4-track steel approach trestle about 600 feet long which connects
with the dock.
In adopting a design for the LS&I dock, it would be nice to think
the engineers planned for the future size of lake ships. However, the 1200-foot-long dock
was probably constructed to load three ships on each side. The 1200-foot length does allow
the load of modern 1,000-foot vessels. The original dock was built to load vessels of a
maximum width of 50-60 feet, while today’s 1,000 footers are 105 feet wide.
Therefore, to fully load these larger vessels, they must go out into the harbor and turn
around so that the remainder of the cargo can be placed!
The annual shipping capacity of the dock is estimated at 9.5 to 10
million tons of ore. The largest annual shipment was 9,465,601 tons in 1989. The average
tons of each vessel loaded was 24,113 in 1998 compared with 2,431 tons in 1899.
Source: Unknown
Source: Unknown
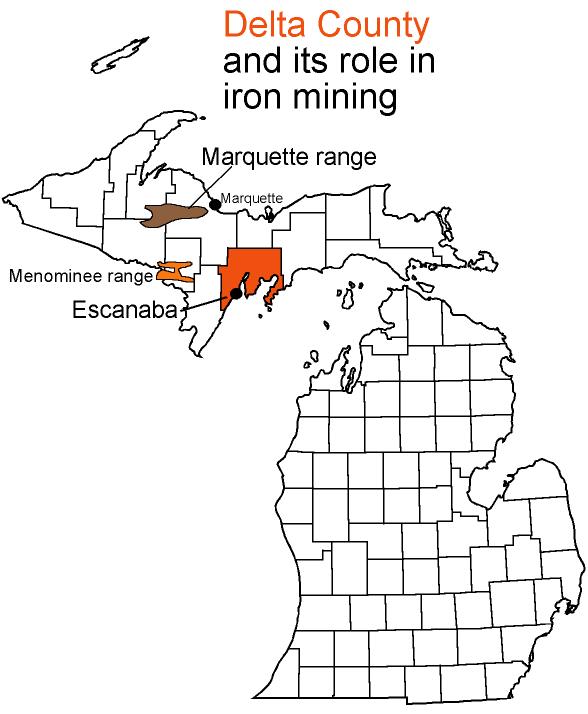
Delta County has played a large role in Michigan’s once great iron ore mining
industry.
Marquette and Escanaba have, traditionally, been the main ports through which iron ore has
been shipped (see map below).
Source: Unknown
Escanaba has the advantage of being useable year-round; Marquette must be shut down in
winter when the Soo Locks are closed. However, Marquette has the advantage of being
closer to the main mine sites. In winter, most ore is taken by train to Escanaba and
shipped out of there. In summer, the reverse is true.
Source: Unknown
Like other Upper Peninsula counties, Delta has a long record of lumbering and fishing. But
its excellent natural harbors and proximity to the iron ranges give it a special place in
Michigan’s iron mining history.
Because the Ojibwa words that sound like Escanaba denote either
"land of the red buck" or "flat rock," doubt remains about the
name’s meaning. But there is little doubt about the important role Escanaba has
played in Michigan’s mining industry. In 1864 the Peninsula Railroad (now part of the
Chicago and North Western) was completed from Negaunee to Escanaba. At the same time, iron
ore loading docks were built at Escanaba.
With the completion of both projects, iron ore from the Marquette Range
near Negaunee could be transported by rail to Escanaba then shipped to steel mills along
the Great Lakes. The first shipments, 975 tons bound for Cleveland, left in 1865. In the
late 1870s a rail line was laid from Escanaba westward tapping the iron ore reserves mined
in the Menominee Range. Soon Escanaba, the only iron port on Lake Michigan, bustled with
shipping activity. By the turn of the century Escanaba, which occasionally exceeded
Marquette in the amount of ore shipped annually, had proclaimed itself the Iron Port of
the World.
Though Escanaba long ago lost any claim to iron port of the world, in
its first century over 325 million tons of ore were shipped from its docks.
Michigan iron ore mining declined in the 1920s and 1930s, but its
reactivation with the development of new processes in the 1950s and Escanaba’s
proximity to Chicago’s steel industry have kept iron ore exportation an integral part
of the city’s economy. Today approximately 60% of the iron ore mined in the Marquette
Range, the only Michigan area still mined, is shipped out of Escanaba.
Present-day iron mining in the UP
All of the Marquette Range ore currently mined is excavated from open pits using
diesel-powered off-road trucks loaded by electric and diesel-powered shovels. The ore,
which in the ground consists of 25 to 45 % iron by weight, is pelletized at processing
plants adjacent to each mine. First ground to a fine powder, the ore is next concentrated
using magnetic separation and flotation to 60 to 65 % iron, then rolled into 3/8" dia
pellets that are purplish-grey when the emerge, steaming, from the plants. The rust color
of pellets that have laid exposed to the weather is entirely natural, as the iron in the
pellets is chemically combined with oxygen to form iron oxide, more commonly known as
rust. Small amounts of silica, alumina, manganese, limestone and bentonite make up the
rest of the pellet. Bentonite (an extremely sticky clay) is added at the rate of 16 lbs
per ton as a binder. Limestone, which serves as a flux in the steel-making process, is
added to the pellets at the processing plants to simplify blending at the steel mills. The
limestone comes in on inbound ore boats to both Marquette and Escanaba. At Marquette, it
is offloaded at the Upper Peninsula Power Plant adjacent to the LS&I ore dock and
hauled by truck to the Empire and Tilden Mines. The Chicago & Northwestern Railroad
hauls the limestone unloaded at Escanaba to the Empire Mine in ore cars.
At present, the Empire and Tilden Mine are
the only active iron mines on the Marquette Range. The Empire Mine, owned by Inland Steel,
LTV Steel, Wheeling-Pittsburgh Steel and Cleveland-Cliffs, mines magnetite averaging 34%
iron. The existing mine was opened on the site of the Volunteer Mine in 1964 and greatly
enlarged in 1966, 1975 and 1980, which increased pellet production from 1.6 million tons
to 8.0 million tons annually. Most of the Empire Mine’s production until recently was
hauled out by C&NW to its dock at Escanaba, Michigan, where it is loaded on ore boats
for a southbound trip on Lake Michigan to LTV Steel and Inland Steel at Gary, Indiana.
Cleveland-Cliffs’ pellets are sold on the open market and may go to a variety of
steel mills.
The Tilden Mine was first opened in a large-scale fashion in 1972, when
the open pit was greatly enlarged. The current Tilden Mine is owned by Algoma Steel,
Stelco and Cleveland-Cliffs. The mine is reached by an LS&I branch from Empire Mine
built between1972 and 1974. Production from the Tilden pellet plant began in December
1974; the facility was doubled in size in 1979 and was enlarged by adding a new pit in
1989. Currently, the Tilden ships about 7.0 million tons annually.
All of the underground mines that for over a century were the backbone
of Marquette Range production have long since closed and it is highly unlikely that they
will ever reopen. The Tracy (underground) Mine closed in 1971. Its buildings are still
visible just to the west of Palmer Line Junction. Trackage still runs through the weeds
and the saplings into the mine.
Most visible of the closed-up workings are those of the processing
plants supported by the Mather Mine’s B Shaft. The B Shaft was the last active
underground mine on the Marquette Range prior to its closure in July 1979.
The other two major mines of recent history, one of which may someday
reopen, are the Republic Mine and the Humboldt Mine. The Republic, which dates to 1871,
was idle after 1937 but was reopened in 1954. Pellets were produced at the Republic Mine
beginning in 1962. In the 1960s the Republic Mine was the largest single source for ore on
the Marquette Range. All operations at the Republic Mine and the Eagle Mills Pellet Plant
were temporarily suspended in October 1981, but the mine and pellet plant are intact and
could be reopened should market conditions improve. Experimental research ongoing at the
Eagle Mills Pellet Plant centers on the development of a new "lightweight"
pellet the size of a tennis ball that reportedly is bonded with carbon. The Humboldt Mine
was opened in 1954 to develop a low-grade jasper formation. The Humboldt commenced pellet
production in 1960 and expanded its operations in 1962 and 1963. Its ore reserve was
depleted by the early 1970s, thus after running Republic ore for awhile its pellet plant
closed in 1980. The Humboldt pellet plant has since been sold to the Callahan Mining
Company, which uses it to process small lots of gold ore from the Ropes Gold Mine north of
Ishpeming.
The decline of copper and iron-ore mining was a
serious economic setback for the Upper Peninsula, but with the development of new mining
and processing methods, this mining industry has recovered somewhat. The employment high
in iron-ore mining was 6,972 in 1952.
Bog iron
At one time a flourishing iron industry was based on the bog iron ore found
in the swamps of southern Michigan. The bog ore and clay-iron-stone concretions found in
the Coldwater shale nearby supplied several furnaces in Union City- the first iron
manufactured in Michigan. Bog iron ore (red ocher) was once mixed with water and used as a
cheap paint for the old red barns and the vanished little old red schoolhouse. It has an
important but undeveloped use today as paint filler.
Some of the images and text on this page were taken from various issues of Michigan History magazine and/or have been paraphrased from C.M. Davis’ Readings in the Geography of Michigan (1964).
This material has been compiled for educational use only, and
may not be reproduced without permission. One copy may be printed for personal
use. Please contact Randall Schaetzl (soils@msu.edu)
for more information or permissions.
.