A huge copper boulder on the Ontonagon River, said by Alexander Henry (the first white man to describe it) to weigh five tons led, in 1770, to the first copper mining venture in the UP. This first mining venture started near the boulder that lay 20 miles from the mouth of the Ontonagon River. The "Ontonagon Boulder" was eventually shipped to the Smithsonian Museum and now resides by the Mall Entrance to the Museum of Natural History. Michigan has been trying to get our Boulder back, but the negotiations are a bit slow. You know what happens when things go to Washington. It is about the size of a VW Bug and weighs about 5 tons.
Mining started in 1844 by the sinking of two shafts near Copper Harbor, but both were unsuccessful. Profitable mining began in 1845 when at the Cliff mine the first mass of native copper in place was discovered. Other mines were opened along the extent of the copper bearing rocks; some were exhausted in a short time. Others continued mining, going deeper and deeper until some of the mines are over a mile in vertical depth and several shafts are over 9,000 feet deep on the incline. The copper became harder and more expensive to mine until finally, when cheaper copper could be obtained from other regions, it became unprofitable to mine copper in Michigan. Michigan’s mines, although obtaining pure copper, could not compete with those western mines which obtained copper ores near the surface.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan
State University
From 1847 to 1887, Michigan was the nation’s largest producer of
copper, and as copper production grew, the population of the Upper Peninsula, particularly
the Keweenaw Peninsula, grew with it. The towns of Houghton, Hecla, and Calumet, among
others, were all copper towns peopled by workers employed in the mines. The Cornish, in
particular, left Cornwall in droves during the 1840s and brought their ethnic traditions
and deep-mining techniques to the mines on the Keweenaw Peninsula.
By 1860, three major copper-producing regions had been developed, all
on the Keweenaw, all utilizing shaft mines, and almost all financed by Boston capitalists.
Of these areas, only the Portage Lake are near Houghton was still active by the 1880s. It
was here that the world-famous Calumet and Hecla mines were located; here too, the 1916
war boom sparked a production peak worth an estimated $76 million.
The copper ore-rich rocks occurred in rock layers that dipped steeply beneath
Lake Superior. The dip of the rock layers can be easily envisioned in two
ways. First, the "slant" of the copper mine headframes (seen below)
exactly paralleled the slope of the rock layers, so that copper "man cars" (see
below) could descend into the mine, from the top of the headframe, at a constant
angle. Also, the ore cars that came up, out of the mine also could ascend into the
headframe without a change in angle.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan
State University
Second, the dip of the rock layers can be seen directly in the mine proper. A photo
of a copper mine shaft is shown below, and if you look closely, you will see the steep dip
of the shaft through the circular hole. The image is meant to illustrate that the
dip of the rock layers was much steeper than is shown in the diagram above; in fact, in
most instances the shafts were so steep that standing was almost impossible.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan
State University
Because the dip of the mine shaft is difficult to see, the image below has been enhanced
to show the steepness of the shaft and hence, the dip of the ore body.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan
State University
If you had a hard time seeing the slope (dip) of the mine shaft in those two pictures, try
this one (below):

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan
State University
In some cases, the mines and mine shafts were so long that they dipped well out beneath
the lake as well. Many mines, which started in the center of the Keweenaw Peninsula,
end up well out beneath the lake. Recall that most of the mines were located on the
top of the Keweenaw Range, in more-or-less the center of the Keweenaw Peninsula.
(See maps below.)
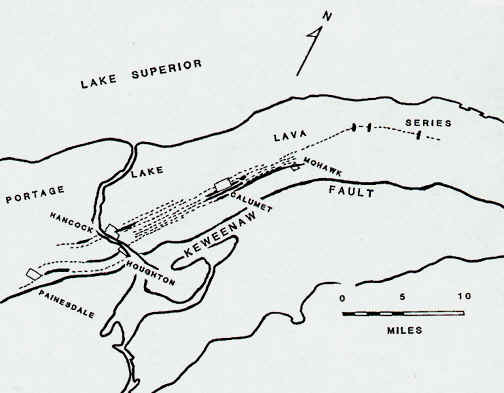
Source: Unknown
Evidence that the copper-bearing solution was hot comes from the widespread
deposition of calcite, quartz, and a variety of zeolite minerals along with the copper.
Since some zeolites form only at elevated temperatures, the solutions must have been hot,
perhaps 200o C or more. Since higher temperatures increase the solubility of
all substances, copper would be more soluble at higher temperatures and less soluble as
the solutions cooled. Thus, cooling of the solutions could have caused precipitation of
the copper.
In places native silver is found either intergrown with the copper or
as independent masses or crystals. Mineral collectors avidly seek out such specimens.
While there is not sufficient silver to warrant mining, its presence in the copper
increases the value of the ore mined.
Once at the surface, the metallic copper had to be
removed from the lava ore that surrounded it. Michigan copper is so
pure (and in metallic form) that removal of the copper could be done by
"stamping" the ore in large stamp mills, such as the one shown below.
Today, the ruins of one large stamping operation is being restored as part of the Keweenaw National Historic Park. The
stamp rock, containing minute quantities of finely divided copper and copper ore, was
dumped as tailings.

Source: Photograph by Randy Schaetzl, Professor of Geography - Michigan
State University
The stamp mills would crush the ore, allowing for easy removal of the metallic copper.
The refuse--basaltic lava ground to sand size, was discarded. Much of the
discarded "stamp sands" were placed in the Houghton-Hancock ship canal, filling
large parts of it up.
Ever heard the term "Float Copper"?
You might get a startled expression from people when you mention "drift
copper" or "float copper." They think: "Copper is dense - how can it
float or drift? What is it floating or drifting on?" Drift refers to glacial drift,
which is any sediment deposited as a direct or indirect result of glaciation. Copper
nuggets carried by glaciers and meltwater rivers is called "drift copper."
Float is a geological term used to denote any material that has been carried by
erosion away from its spot of formation. A slab of rock slides down a hill. It is now
float. A glacier carries a rock a hundred miles and drops it as it melts. That rock is now
both drift and float, whose source is "up glacier" somewhere. Thus, in the
Copper Country, "drift copper"and "float copper" refer to the same
thing.
Floating minerals leave trails a good prospector can patiently trace
back to their source. For example, gold panners will pan their way upstream to the
"Mother Lode". Examination of float diamonds and other kimberlite minerals has spurred exploration recently in
Michigan and Canada. The Native Americans used this technique to first find the source of
the Midwest copper deposits.
Its no mystery today where most Midwest drift copper originates. Native
copper is abundant in the Upper Peninsula of Michigan in basaltic lava and interlayered
sediments formed approximately a billion years ago. Much smaller deposits are known where
these same rocks extend into Wisconsin and Minnesota. Freed from the rock by weathering,
this copper can survive long transport by glaciers and rivers because of its tenacity and
relatively low chemical reactivity. Float copper is found in all states that have received
glacial drift from the Lake Superior region. It is easily recognized by its bright green
to black alteration crust (consisting of malachite, cuprite and other minerals), high
density, malleability and brilliant copper color on a fresh surface.
Rising prices and increased competition forced the smaller mining companies to close or to
consolidate, but by 1936, even the two remaining giants, Calumet and Hecla Consolidated
and the Copper Range Company, could no longer compete with the newer and cheaper western
copper. The economic success of Michigan mines had been based on an inexpensive extraction
of high-grade ores. As sources were depleted and quality ore became harder and harder to
obtain, these copper mines lost their national prominence.
The White Pine Copper
Deposit. The problem in Michigan copper mines is not one of the ore
depletion---the lodes show no lessening of value with depth---nor related to the
geological occurrence of the metal, but a problem of ability of the producers to compete
with foreign and western production. The White Pine deposit, also Precambrian, is a
sulfide copper rather than a native copper. The rocks are fine-grained, and copper is only
one of the minerals that compose the rock.
The White Pine copper deposit is located near White Pine, Michigan,
about 70 km southwest of the main native-copper producing area. It is in the Iron River
syncline, just east of the Porcupine Mountains. The ore at White Pine is in the Nonesuch
Shale, and consists mainly of the copper sulfide mineral chalcocite (Cu2S).
Although the presence of copper mineralization in the White Pine area
has been known since about 1850, successful mining operations on a large scale began in
1953, and ended in 1999. Early attempts at mining the copper in the Nonesuch Shale were
small-scale, generally unsuccessful operations. It was not until technological advances
made it possible to recover the chalcocite that major development of the ore body was
undertaken. Since then it has been a major producer of copper in the United States, with
one billion pounds of copper recovered in the first 10 years of operation.
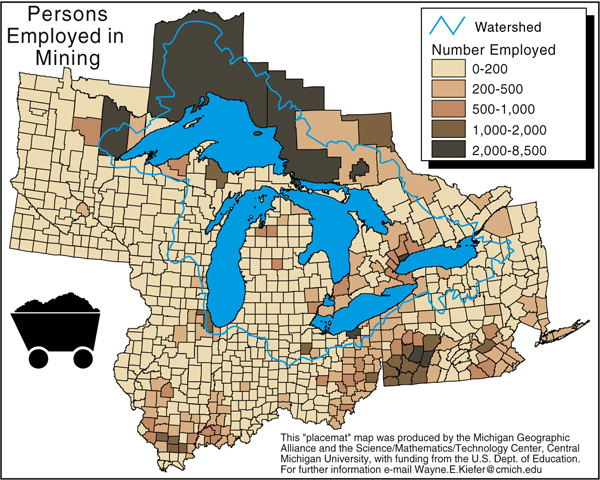
As the map below shows, copper mining is almost nonexistent in the Keweenaw today. The White Pine mine, located in Ontonagon County, may be the only really prosperous mine in the area.
Silver is a generous byproduct of the copper mines. Its presence in the copper in
microscopic flakes makes Michigan’s copper premium metal. Free silver has been found
near Ontonagon. Of interest rather than commercial value are the semi-precious gem stones,
chlorastrolite, thompsonite, carnelian, agate, found along the beaches of the copper
counties and Isle Royale. The trap rock of the Copper Country, like the traps of the
remainder of the Precambrian area, are also a valuable resource for road material.
Some of the images on this page were taken from an issue of Michigan History magazine and from C.M. Davis’ Readings in the Geography of Michigan (1964). Some of the other images were donated by Nate Verhanovitz, a mechanical engineering grad. student at MSU.
This material has been compiled for educational use only, and may not be reproduced without permission. One copy may be printed for personal use. Please contact Randall Schaetzl (soils@msu.edu) for more information or permissions.